ADDISON LAB
≫ PROFILE
Dr. Addison is an Assistant Professor at Kyushu Dental University in the Division of Molecular Signaling and Biochemistry. He received his B.Sc. degree in Anatomy and Cell Biology from McGill University (Montreal, Canada) in 2004, and a Ph.D. degree from McGill University in 2010. His Ph.D. work was focused on molecular determinants of extracellular matrix mineralization. Following this, Dr. Addison moved to Harvard University (Boston, USA) where he would spend 5 years as a postdoctoral fellow exploring transcriptional dynamics during fate specification of mesenchymal stem cells. Dr. Addison then studied gene expression in bone cells as a postdoctoral research scientist at the Shriners Hospital for Children Canada and McGill University from 2016 to 2019 before joining Kyushu Dental University in 2019.
≫ LAB MEMBERS
AKINO GOTO (後藤 晶乃):[Graduate Student, 大学院生]
CHIRADA DUSADEEMEELAP:[Graduate Student, 大学院生]
≫ OUR RESEARCH
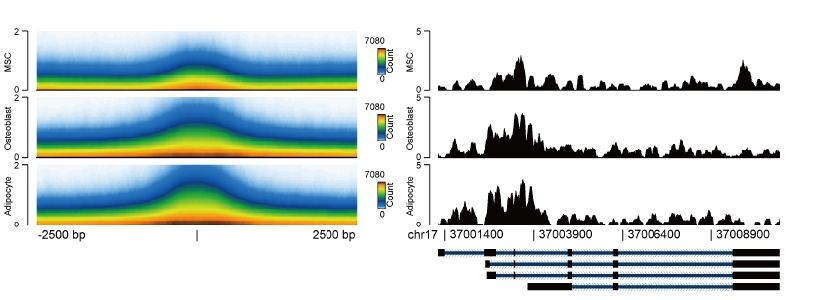
Our lab studies the molecular mechanisms underlying transcriptional and epigenetic control of lineage commitment in mesenchymal stem cells.
This research has implications for osteoporosis, sarcopenia, obesity and other musculoskeletal diseases.
Mesenchymal stem cells can differentiate into a variety of lineages, including bone, fat, muscle, and cartilage. This provides a unique opportunity to study regulatory events that control cellular transitions and lineage commitment. Lineage-specific remodeling events and epigenetic dynamics frequently precede transcriptional activation. A better understanding of these events will lead to novel medical applications impacting diseases in which imbalances in lineage commitment occur. Furthermore, we aim to develop strategies for the rewiring or reprogramming of transcriptional networks towards engineering cell-based therapies for musculoskeletal disease.
Our research themes:
A) Epigenetic abnormalities in craniofacial defects
B) Chromatin dynamics in skeletal stem cells
C) Orofacial muscle injury models
D) Molecular determinants of bone mineralization
To address these aims, we use a variety of interdisciplinary approaches including cell biology, molecular biology, mouse genetics, tissue engineering, biochemistry proteomics and advanced imaging. Our research provides a strong scientific basis for the training of researchers and clinical scientists. We are interested in recruiting highly motivated, ambitious and talented trainees.
Keywords: gene expression, transcription, epigenetics, molecular biology, osteoblast, adipocyte, myoblast, differentiation, stem cells, mineralization.
External Sites:
[ORCID Profile] https://orcid.org/0000-0002-8587-8028
[PUBMED Bibliography] https://www.ncbi.nlm.nih.gov/myncbi/william.addison.1/bibliography/public/
≫ SELECTED PUBLICATIONS
Hariri H, Addison WN, St-Arnaud R. Ubiquitin specific peptidase Usp53 regulates osteoblast versus adipocyte lineage commitment. Sci Rep. 2021 Apr 19;11(1):8418.
Addison WN, Pellicelli M, St-Arnaud R. Dephosphorylation of the transcriptional cofactor NACA by the PP1A phosphatase enhances cJUN transcriptional activity and osteoblast differentiation. J Biol Chem. 2019 May 17;294(20):8184-8196.
Addison WN, Hall KC, Kokabu S, Matsubara T, Fu MM, Gori F, Baron R. Zfp423 Regulates Skeletal Muscle Regeneration and Proliferation. Mol Cell Biol. 2019 Apr 15;39(8).
Addison WN, Nelea V, Chicatun F, Chien YC, Tran-Khanh N, Buschmann MD, Nazhat SN, Kaartinen MT, Vali H, Tecklenburg MM, Franceschi RT, McKee MD. Extracellular matrix mineralization in murine MC3T3-E1 osteoblast cultures: an ultrastructural, compositional and comparative analysis with mouse bone. Bone. 2015 Feb;71:244-56.
Addison WN, Fu MM, Yang HX, Lin Z, Nagano K, Gori F, Baron R. Direct transcriptional repression of Zfp423 by Zfp521 mediates a bone morphogenic protein-dependent osteoblast versus adipocyte lineage commitment switch. Mol Cell Biol. 2014 Aug;34(16):3076-85.